
Introduction: From proof of concept to factory, a path full of challenges
In the field of biotechnology, transitioning from a successful laboratory experiment to large-scale production is not simply a matter of switching to a bigger vessel. This process, known as scale-up, is a key step that often brings its share of surprises. It requires not only specific skills and expertise, but also state-of-the-art equipment. Furthermore, it must take into account strict regulations as well as economic and regulatory constraints.
Transitioning from a successful laboratory experiment to large-scale production is not simply a matter of switching to a bigger vessel. This process, known as scale-up, is a key step that often brings its share of surprises. It requires not only specific skills and expertise, but also state-of-the-art equipment. Furthermore, it must take into account strict regulations as well as economic and regulatory constraints.
So what exactly is scale-up? What is its purpose? What are the key stages, challenges, and risks ? This article explains everything you need to better understand this key stage of industrialization in biotechnology.
What is “scale-up” in biotechnology ?
Scale-up refers to all the operations aimed at reproducing a process developed in the laboratory—often using small bioreactors (ranging from a few milliliters to a few liters)—under conditions that do not yet reflect the reality of industrial production.
This stage involves both the production process in fermenters (“Upstream Processing”) and the treatment of the product obtained after cultivation (“Downstream Processing”).
In biotechnology, this applies for example to:
- microbial fermentation (involving bacteria, yeasts, or fungi),
- cell culture (mainly plant or animal cells),
- biochemical or enzymatic transformation processes, such as the hydrolysis of cellulose or lactose.
The goal ? Achieve the same technical performance and product quality at volumes of hundreds or thousands of liters… which is far from straightforward.
Why is scale-up so critical ?
Contrary to popular belief, scale-up isn’t just about multiplying volumes. Physico-chemical conditions, flow rates, residence times, and heat or mass transfer do not scale linearly. This makes scale-up a multidimensional change, with a major impact on:
- Reproducibility and robustness of the process,
- Yield,
- Final product quality,
- Economic viability of the process.
What are the main stages of scale-up?
Scale-up typically follows a phased approach, with scale “milestones” to validate performance at each level.
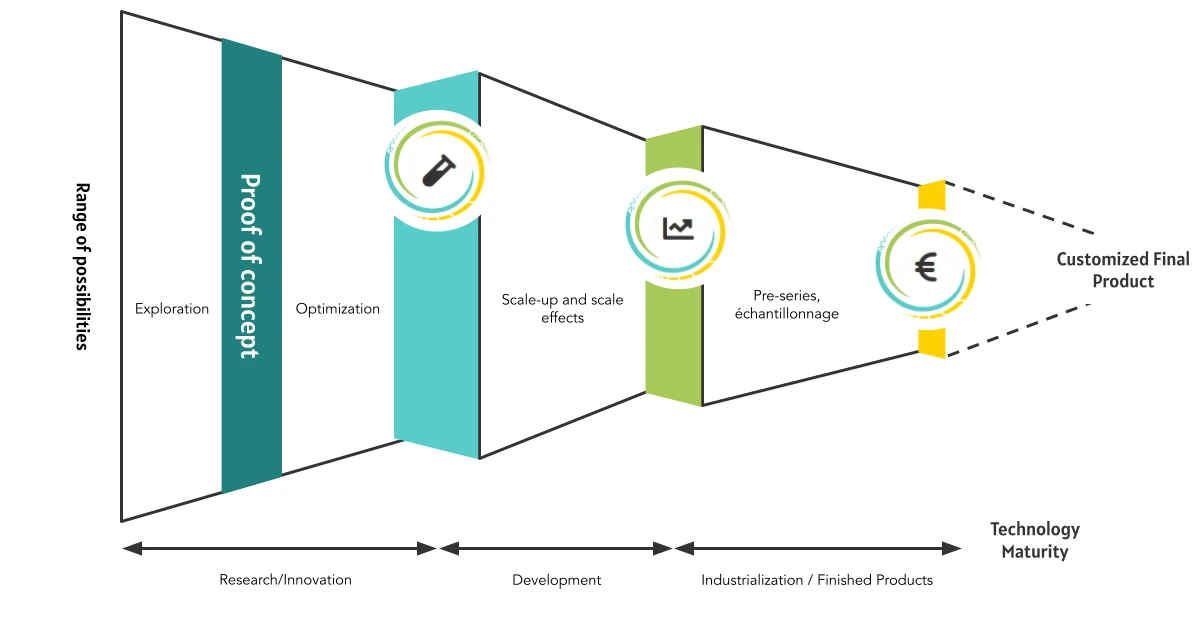
1. Laboratory Phase (Proof of Concept)
- Scale: milliliters to a few liters
- Objectives:
- Validate a biological principle (strain, enzyme, substrate, etc.)
- Optimize key parameters (pH, temperature, time, etc.)
- Define the selection of technologies used for Downstream Processing (DSP)
- Tools: lab-scale bioreactors, detailed yield analysis
2. Pilot Scale-Up
- Scale: 50 to 1,000 liters (depending on the process)
- Objectives:
- Test the robustness and transferability of the process,
- Identify the key points and bottlenecks in the production process,
- Adjust fermentation parameters (mixing, oxygen transfer, etc.) that differ significantly from laboratory conditions,
- Reduce the risk of contamination and test different process management strategies, while allowing for rapid modifications based on the results obtained,
- Acquire valuable expertise on the behavior of the process at large scale, thus facilitating the transfer to industrial production,
- Produce sufficient biomass or product to carry out downstream processing trials using pilot-scale tools representative of industrial technologies,
- Validate the technical and economic feasibility of the complete process and produce samples for application testing
- Tools: technology halls with modular equipment
3. Pre-Industrialization
Scale: 1,000 to 10,000 liters and more
Objectives:
- Verify the robustness and reproducibility of the process,
- Prepare the regulatory dossiers,
- Produce test batches (pilot series).
- Tools: semi-industrial platforms, industrial simulators
4. Full Industrialization
- Scale: continuous or batch industrial production
- Objectives:
- Produce at a controlled cost
- Comply with quality and safety standards
- Ensure integration into the supply chain
What are the main technical challenges of scale-up ?
Scale-up in biotech is a balancing act between biology, engineering, and economics. Here are the main challenges R&D and process teams face:
Maintaining biological performance
- Cell growth, protein expression, or enzymatic yield can drop at scale
- Risks linked to oxygenation, mixing (gradient formation), mechanical stress
Managing transfer phenomena
- Heat transfer: large volumes pose cooling challenges
- Mass transfer: aeration, mixing, homogeneity
Adapting purification steps
- Downstream processing (filtration, centrifugation, chromatography…) must be compatible with the volume, without any loss of quality and with minimal material loss.
Optimizing costs
- The process must be profitable at scale: raw materials, energy, maintenance…
- Techno-economic simulation is essential
How to de-risk a scale-up project ?
Given its complexity, several levers can help reduce risks and improve efficiency:
Collaborate with a technology hall
Having access to a fully equipped pilot environment such as that of ATV offers numerous advantages.
Not only does it provide access to modular semi-industrial equipment, but it also allows you to leverage specialized expertise to carry out trials under conditions close to actual production.
This stage offers the opportunity to adjust and optimize processes while avoiding the heavy investments associated with building a complete factory.
Conduct early techno-economic studies
Analyzing profitability, operational costs, and regulatory feasibility from the pilot phase is key to:
- Convincing investors
- Guiding technology choices
- Preparing for the industrial phase
Rely on a multidisciplinary team
A successful scale-up requires tight coordination between biologists, process experts, automation experts, quality and regulatory specialists, etc. Solid project management and fluid communication are vital.
Real-life examples of successful scale-ups
To illustrate, here are two anonymized case studies from projects carried out in a technology hall:
Case #1 – Cosmetic ingredient via fermentation
- Objective : Develop the production process from laboratory scale (5 L bioreactor) to production scale (4,000 L bioreactor)
- Challenges: Maintaining yields and productuctivity, transferring the process to industrial conditions (culture medium, sterilization, oxygen transfer, etc.).
- Outcome: Reduction of time-to-market by 9 months, validation by a major European group.9-month reduction in time-to-market, validated by a major European group
Functional protein for animal feed
- Objective : Industrialize an enzymatic process developed at the start-up scale.
- Challenges: Achieving regulatory compliance and reducing purification costs.
- Outcome: Production of an industrial batch enabling successful fundraising.
Conclusion: An essential step in scaling innovation
Scale-up isn’t just about tanks and pumps. It’s a discipline in its own right, at the crossroads of science, engineering, and industrial strategy. If poorly handled, it can doom even the most promising projects. But when well executed, it can transform a lab innovation into a tangible, competitive, and sustainable product.
At ATV, we support startups and industry players through this crucial step from R&D to industrialization, thanks to our expertise and our 7,000 m² technology hall. Want to learn more about our support ? Contact us here.

Prêt à passer à l’échelle avec ATV Technologies ?
Guillaume, notre ingénieur commercial, vous présente notre offre, analyse vos besoins et constitue l’équipe projet idéale pour concrétiser votre innovation.